Pembrolizumab-Induced Optic Neuritis: A Case Report
Abstract
Immune checkpoint inhibitors (ICIs) have transformed the treatment of relapsed and refractory Hodgkin's lymphoma, but they are associated with immune-related adverse effects (irAEs). Although rare, ocular irAEs can have a significant impact on vision. This is the case of a 29-year-old female with relapsed classical Hodgkin's lymphoma, who experienced unilateral, painless vision loss after two cycles of pembrolizumab-based therapy. The MRI revealed optic nerve inflammation and extensive investigations ruled out other causes. A diagnosis of immune-related optic neuropathy was made. Pembrolizumab was stopped, and high-dose intravenous methylprednisolone was immediately started, followed by a six-week prednisolone taper, which resulted in complete visual recovery within two weeks. The patient achieved remission with DHAP chemotherapy before receiving autologous stem cell transplantation. This case emphasises the necessity of early identification and prompt corticosteroid treatment for ocular immune-related adverse events, underscoring the requirement for meticulous surveillance and swift action to avert permanent vision impairment.
Keywords: Case Report, Optic Neuritis, Pembrolizumab
Background
Immune checkpoint inhibitor (ICI) treatment has established its relevance in refractory and relapsed Hodgkin’s lymphoma.[1,2] ICIs are effective but can lead to immune-related adverse events (irAEs) due to overstimulation of the immune system.[3] Ocular irAEs are rare side effects but can have a major impact on the quality of life i.e., in the case of impaired vision or complete loss of vision.[3–7] Generally it appears with in few weeks of starting pembrolizumab but delay presentation also been recorded.[8] Ophthalmic irAEs are categorized by the affected area of the eye into ocular inflammation (e.g., uveitis, episcleritis, blepharitis, keratitis), orbitopathy (idiopathic or thyroid-induced orbitopathy), retinal/choroidal disease, and optic neuropathy.[4]
Patient Information
A 29-year-old female with a history of stage IV (Ann Arbor classification) Classical Hodgkin’s Lymphoma (CHL) came to the emergency department with the complaint of painless loss of vision in the right eye since 7 days.
The patient was diagnosed with Stage IV CHL with bone marrow involvement in April 2021, and ABVD regime (Doxorubicin hydrochloride, Bleomycin sulphate, Vinblastine sulphate and Dacarbazine) followed by Escalated BEACOPP leading to remission and a 2-year disease-free survival. In January 2024, patients relapsed with the disease activity in bone marrow. For the same, 2 cycles of P-ICE (Pembrolizumab, Ifosfamide, Carboplatin, Etoposide) were given and the patient received a partial response. After the 7 days of the 2nd cycle, the patient presented with unilateral painless loss of vision.
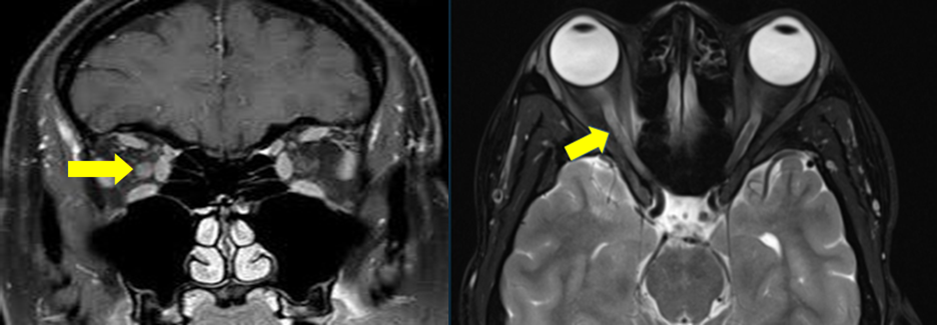
On examination, no light perception in the right eye, fundus examination was unremarkable. MRI showed a bulky right optic nerve with inflammatory changes.[Figure 1] The CSF study was normal. Paraneoplastic panel (including anti-amphiphysin antibodies, anti-Hu/anti-Yo/anti-Ri/anti-Ma/anti-Tr antibodies, anti-SOX1 antibodies, anti-ZiC4 antibodies, anti-collapsin-responsive mediator protein 5 antibodies [CRMP 5], anti-glutamic acid decarboxylase antibodies [anti-GAD], antibodies) were negative.
Considering these diagnostic results, the patient was diagnosed with immune-related optic neuropathy due to PD-1 blockade with pembrolizumab.
High-dose steroid treatment was immediately initiated with intravenous methylprednisolone at a dose of 500 mg per day for three days, followed by tapering of prednisolone over six weeks. The patients showed dramatic improvement with complete recovery of vision within two weeks of steroid treatment. Due to favourable clinical outcome regarding binocular vision, additional treatment was not initiated. The treatment with pembrolizumab was discontinued permanently. Subsequently, the patient was treated with 2 cycles of Dexamethasone, High-dose Ara-C - cytarabine, and Cisplatin (DHAP). The patient achieved complete remission and was posted for an autologous stem cell transplant.
Discussion
To date, ocular adverse events (AEs) following immune checkpoint inhibitors (ICI) have been mostly described either in clinical trials or in retrospective case reports and small series, all of which are limited by small sample sizes.[6] Ocular side effects following ICI treatment are rare, occurring in approximately 3% of patients, as documented in the FDA Adverse Event Reporting System (FAERS) pharmacovigilance database.[3–7] This rare occurrence is thought to be primarily attributable to the immune-privileged location.[9]
The spectrum of ocular irAEs ranges from the most common ocular side effects, including uveitis & keratoconjunctivitis sicca (dry eye syndrome), to optic neuropathy which is much rarer (0.2%).[3–7,10] This case illustrates a rare but significant immune-related adverse event, pembrolizumab-induced optic neuritis, in a patient with relapsed classical Hodgkin’s lymphoma (CHL).
Initiating treatment promptly after diagnosis is essential, with corticosteroids being the main method of therapy. This highlights the importance of being alert to irAEs and the vital role that rapid administration of corticosteroids plays in avoiding permanent loss of vision.
Options for further treatment are mainly based on case series and case reports. In the literature, the following additional interventions are described: plasmapheresis, intravenous immunoglobulin, infliximab, rituximab, and mycophenolate mofetil (MPA).[10–13]
References
- Chen R, Zinzani PL, Lee HJ, Armand P, Johnson NA, Brice P, et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood 2019;134(14):1144–53. doi:10.1182/blood.2019000324.
- Kuruvilla J, Ramchandren R, Santoro A, Paszkiewicz-Kozik E, Gasiorowski R, Johnson NA, et al. Pembrolizumab versus brentuximab vedotin in relapsed or refractory classical Hodgkin lymphoma (KEYNOTE-204): an interim analysis of a multicentre, randomised, open-label, phase 3 study. Lancet Oncol 2021;22(4):512–24. doi:10.1016/S1470-2045(21)00005-X.
- Haanen J, Obeid M, Spain L, Carbonnel F, Wang Y, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Annals of Oncology 2022;33(12):1217–38. doi:10.1016/j.annonc.2022.10.001.
- Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer 2021;9(6):e002435. doi:10.1136/jitc-2021-002435.
- Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. Journal of Clinical Oncology 2021;39(36):4073–126. doi:10.1200/JCO.21.01440.
- Noble CW, Gangaputra SS, Thompson IA, Yuan A, Apolo AB, Lee J-M, et al. Ocular Adverse Events following Use of Immune Checkpoint Inhibitors for Metastatic Malignancies. Ocul Immunol Inflamm 2020;28(6):854–9. doi:10.1080/09273948.2019.1583347.
- Bomze D, Meirson T, Hasan Ali O, Goldman A, Flatz L, Habot-Wilner Z. Ocular Adverse Events Induced by Immune Checkpoint Inhibitors: A Comprehensive Pharmacovigilance Analysis. Ocul Immunol Inflamm 2022;30(1):191–7. doi:10.1080/09273948.2020.1773867.
- Telfah M, Whittaker TJ, C. Doolittle G. Vision loss with pembrolizumab treatment: A report of two cases. Journal of Oncology Pharmacy Practice 2019;25(6):1540–6. doi:10.1177/1078155219841683.
- Zhou R, Caspi RR. Ocular immune privilege. F1000 Biol Rep 2010;2. doi:10.3410/B2-3.
- Francis JH, Jaben K, Santomasso BD, Canestraro J, Abramson DH, Chapman PB, et al. Immune Checkpoint Inhibitor-Associated Optic Neuritis. Ophthalmology 2020;127(11):1585–9. doi:10.1016/j.ophtha.2020.05.003.
- Boisseau W, Touat M, Berzero G, Savatovsky J, Marabelle A, Touitou V, et al. Safety of treatment with nivolumab after ipilimumab-related meningoradiculitis and bilateral optic neuropathy. Eur J Cancer 2017;83:28–31. doi:10.1016/j.ejca.2017.05.036.
- Kim JM, Materin MA, Sznol M, Kluger HM, Weiss S, Chow J, et al. Ophthalmic Immune-Related Adverse Events of Immunotherapy: A Single-Site Case Series. Ophthalmology 2019;126(7):1058–62. doi:10.1016/j.ophtha.2019.01.031.
- Wilson MA, Guld K, Galetta S, Walsh RD, Kharlip J, Tamhankar M, et al. Acute visual loss after ipilimumab treatment for metastatic melanoma. J Immunother Cancer 2016;4(1):66. doi:10.1186/s40425-016-0170-9.
